PDF] Assessment of Hearing Impairment Using Brainstem Evoked Response Audiometry ( BERA ) In Neonates with Various Otonoxious Risk Factors
$ 19.99 · 4.7 (694) · In stock

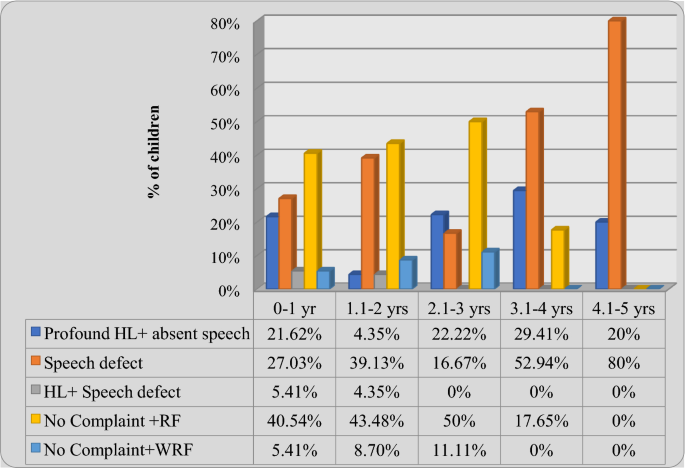
Proportion of newborn with impaired BERA was high in high risk newborn when compaired to general population and Sepsis, very low birth weight and hyperbilirubinaemia in exchange range were found to have significant hearing impairment. Objectives: The aim of this study was to assess hearing impairment in newborn having otonoxious risk factors. Methods: 270 newborns with risk factors for hearing impairment were subjected to BERA initially with90 dB and subsequently stimuli at decreasing frequencies i.e. 75, 60, 45 dB will be presented to each ear at an intensity of 90dB hearing level. An infant will be considered as passed the test if wave V was present at 30 dB in both ears or in one ear at 30 dB and in the other at 45dB. Results: Out of the 270 newborns, BERA was found to be impaired in 48 cases with increased hearing threshold, remaining 222 neonates had normal hearing threshold of 30dB bilaterally and 45dB in one ear and 30 dB in the other ear.Very low birth weight babies with impaired hearing was 25%, hyperbilirubinaemia in exchange range having hearing impairment were 45%, newborns with sepsis and hearing impairment were 32.5%, however after multiple logistic regression analysis sepsis was found to have strong relationship with hearing impairment p value <0.001 and OR 10.991. Elevated auditory threshold was found more frequently in neonates with multiple clinical adverse factors than in those having single risk factor (36/133 Vs 12/137, p <0.001,OR 3.87). Conclusion: Proportion of newborn with impaired BERA was high in high risk newborn when compaired to general population. Sepsis ,very low birth weight and hyperbilirubinaemia in exchange range were found to have significant hearing impairment.

PDF) Correlation between brainstem evoked response audiometry with other audiological tests in different types of hearing loss

Comparison of Otoacoustic Emission (OAE) and Brainstem Evoked Response Audiometry (BERA) in High Risk Infants and Children under 5 Years of Age for Hearing Assessment in Western India: A Modification in Screening

Evaluation of SON'OR©, a Medical Device for Provoked Otoacoustic Emissions and Brainstem Evoked Response Audiometry Made in Cameroon

Comprehensive evaluation of risk factors for neonatal hearing loss in a large Brazilian cohort
Comparison of Otoacoustic Emission (OAE) and Brainstem Evoked Response Audiometry (BERA) in High Risk Infants and Children under 5 Years of Age for Hearing Assessment in Western India: A Modification in Screening

PDF) Automated ABR Screening for Hearing Loss and its Clinical Determinants among Newborns with Hyperbilirubinemia in National Hospital, Abuja, Nigeria

PDF) Hearing screening of neonates at risk
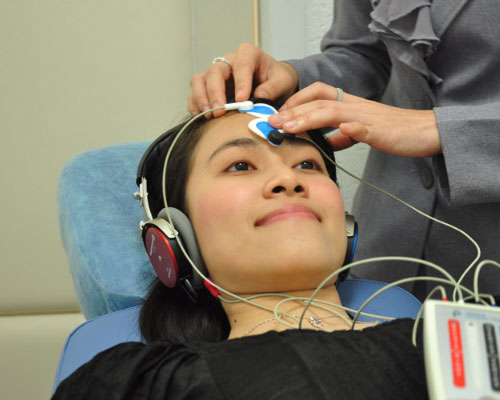
Brainstem Electric Response with Audiometry

Comparison of Otoacoustic Emission (OAE) and Brainstem Evoked Response Audiometry (BERA) in High Risk Infants and Children under 5 Years of Age for Hearing Assessment in Western India: A Modification in Screening

PDF) BERA in detection of hearing loss in children - A Retrospective Study
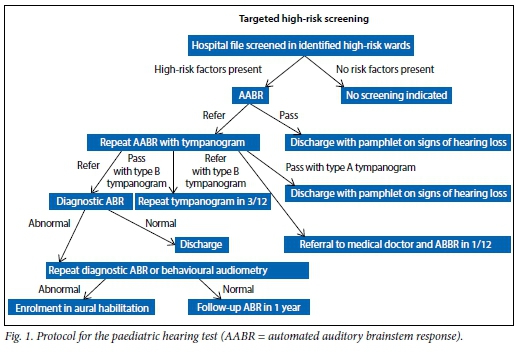
Audiological findings in a group of neurologically compromised children: A retrospective study

210.Brainstem Evoked Response Audiometry BERA Part 2/2 #bera #oae #screening