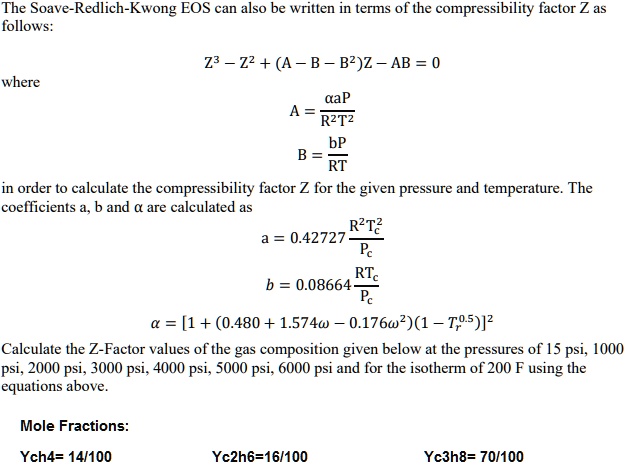
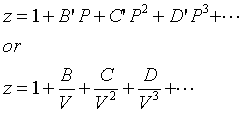Compressibility factor, Z of a gas is given as Z= frac { pV }{ nRT
$ 13.50 · 5 (448) · In stock

Click here:point_up_2:to get an answer to your question :writing_hand:compressibility factor z of a gas is given as z frac pv nrt
Click here👆to get an answer to your question ✍️ Compressibility factor- Z of a gas is given as Z- frac - pV - nRT - -i- What is the value of Z an ideal gas-ii- For real gas what will be the effect on value of Z above Boyle temperature
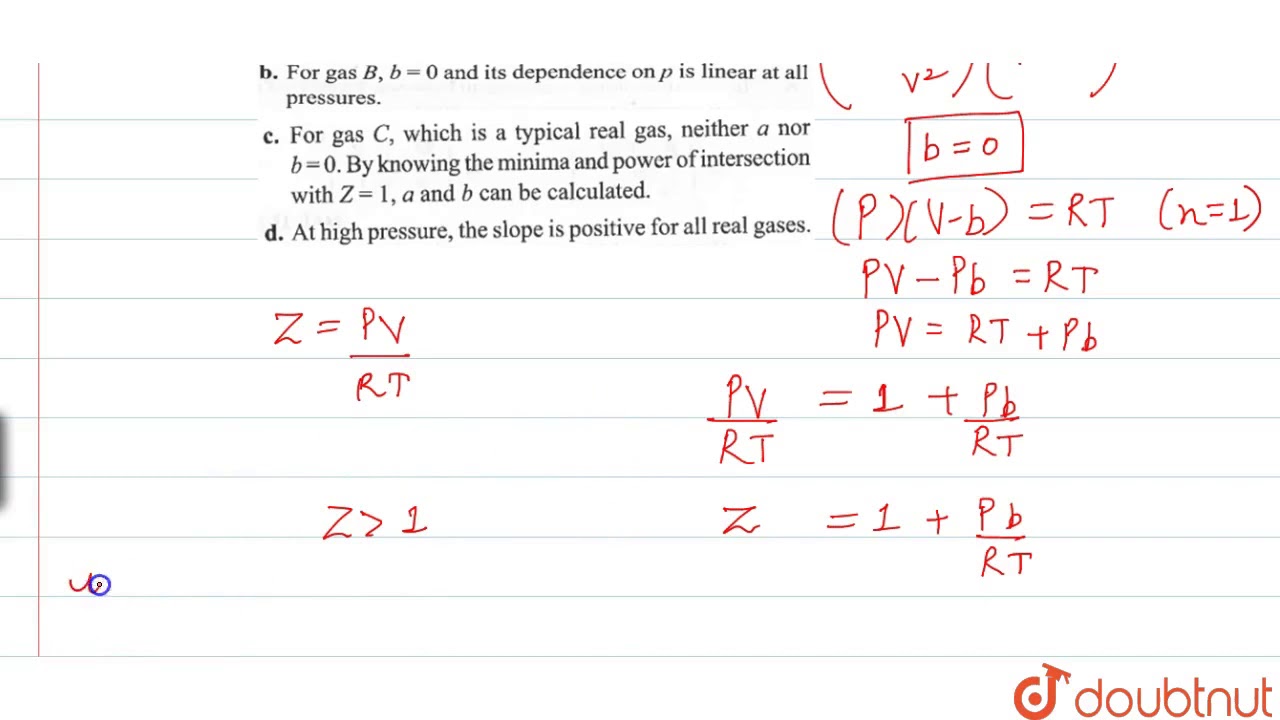
The given graph represents the variations of compressibility factor `Z=PV// nRT` vs `

SOLVED: Derive an expression for the compression factor of a gas that obeys the equation of state p(V - nb) = nRT, where b and R are constants. If the pressure and

Compressibility factor (Z=(PV)/(nRT)) is plotted against pressure

Real gas z-Factor chart [2] Download Scientific Diagram

Compressibility factor `Z=(PV)/(RT)`. Considering ideal gas, real gas, and gases at critical

Energies, Free Full-Text

The compressibility factor of a gas is defined as Z=PV/nRT. The compressibility factor of an ideal gas is:1-1zeroinfinite
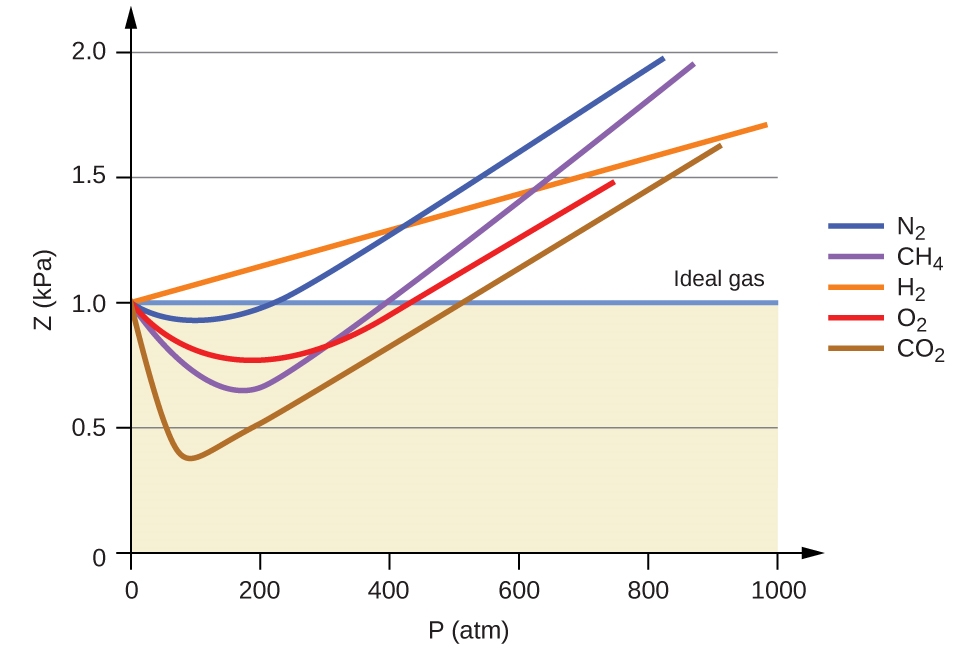
2.8 – Real/Non-Ideal Gas Behaviours – General Chemistry for Gee-Gees

Compressibility factor - Wikipedia
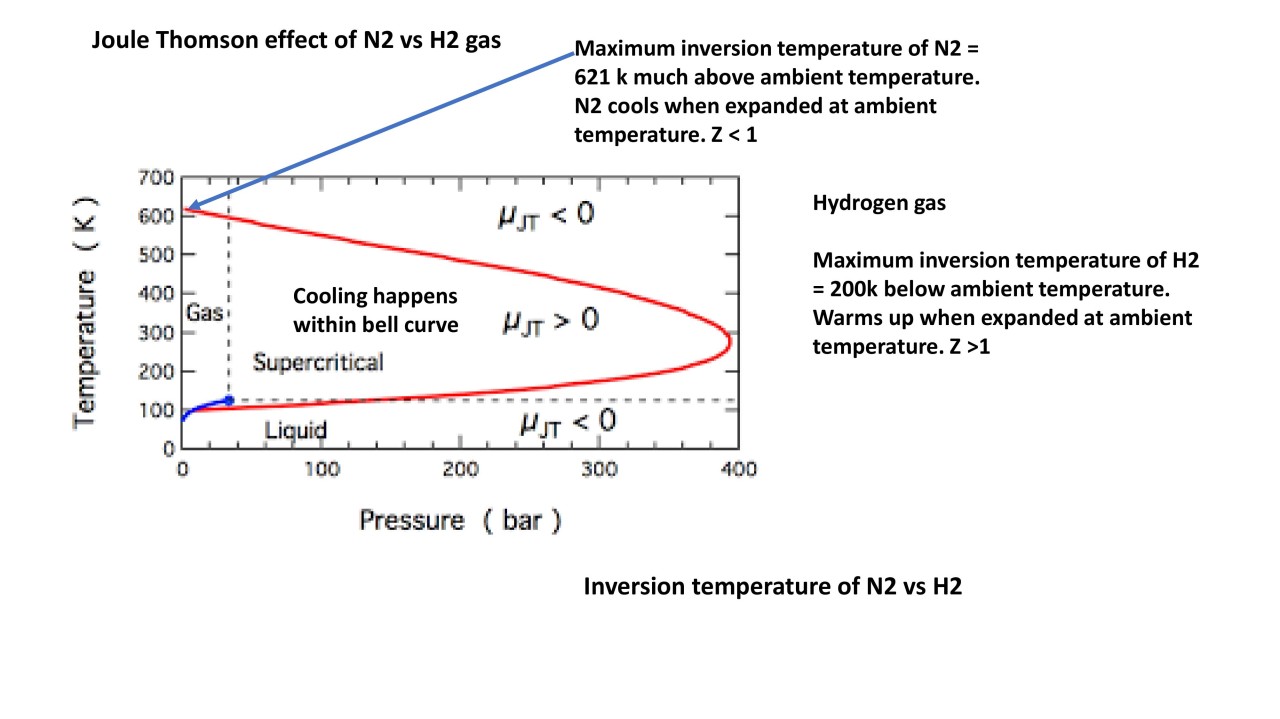
Joule Thomson effect [JT]: A short review

1.7: Connecting the van der Waals and the viral equations: the Boyle temperature - Chemistry LibreTexts
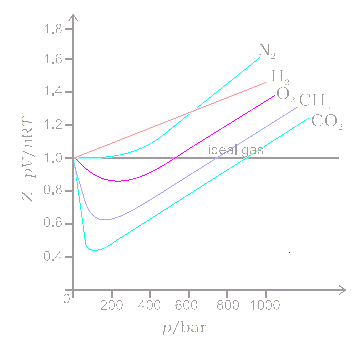
Van der waals equation: Derivation, Explanation

2.8 – Real/Non-Ideal Gas Behaviours – General Chemistry for Gee-Gees

Compressibility Factor of Gas, Overview, Equation & Chart - Lesson