32. 80 g of h2 is reacted with 80 g of o2 to form water. find out
$ 15.50 · 4.8 (647) · In stock
32. 80 g of h2 is reacted with 80 g of o2 to form water. find out the mass of water obtained.which substance is the limiting reagent.
32- 80 g of h2 is reacted with 80 g of o2 to form water- find out the mass of water obtained-which substance is the limiting reagent
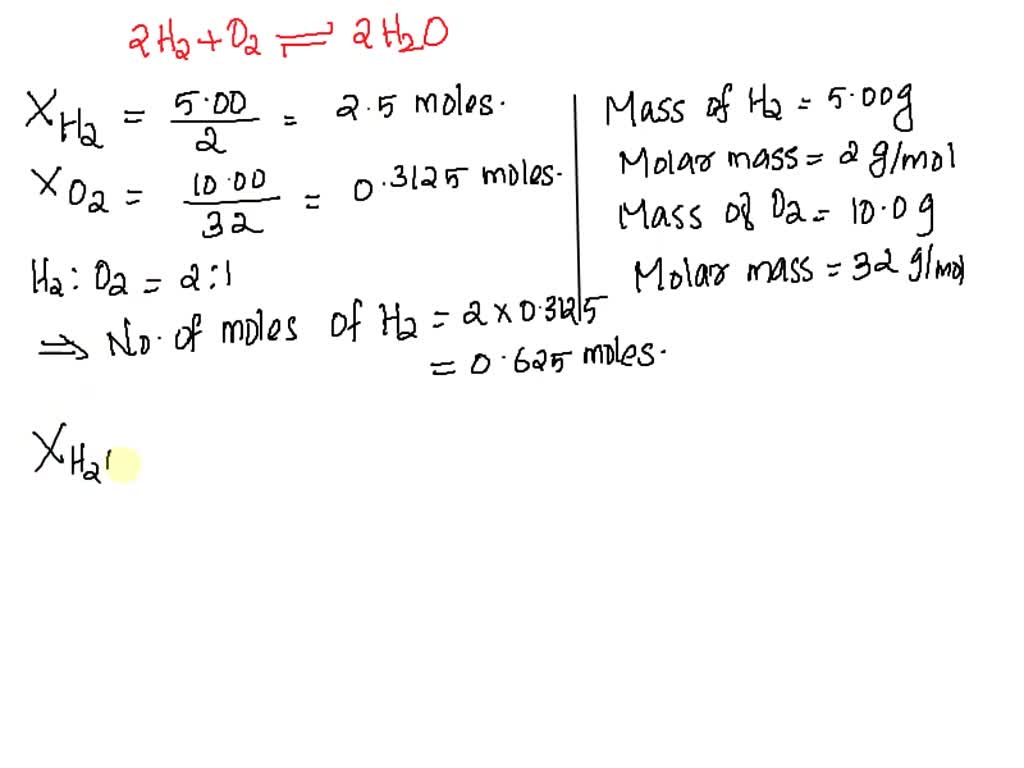
SOLVED: Which is the limiting reactant when 5.00 g of H2 and 10.0

80g of H2 is reacted with 80g of O2 to form water; what are the

13 Reacting Masses

ugures. i 64 of H, reacts with 32 g of Oz to yield water. Which is the limiting reactant? mass of water produced and the amount of excess reagent left. i) Explain
Oxygen, Free Full-Text

20.0 kg of H2 and 32 kg of O2 are reacted to produce H2O.the

2 g H_2 and 1 g O_2 are allowed to react according to following

Hint: N.(g) + 3H2(9) - > 2NH3(9) 28. 80 g of H, is reacted with 80 g of O, to form water. Find out the mass of water obtained. Whia substance is the limiting reagent?
Separating hydrogen and oxygen evolution in alkaline water


:quality(80):fill(white)/https:%2F%2Fimages.asos-media.com%2Fproducts%2Fnew-balance-574-trainers-in-black%2F201068493-1-black%3F$XXL$)





