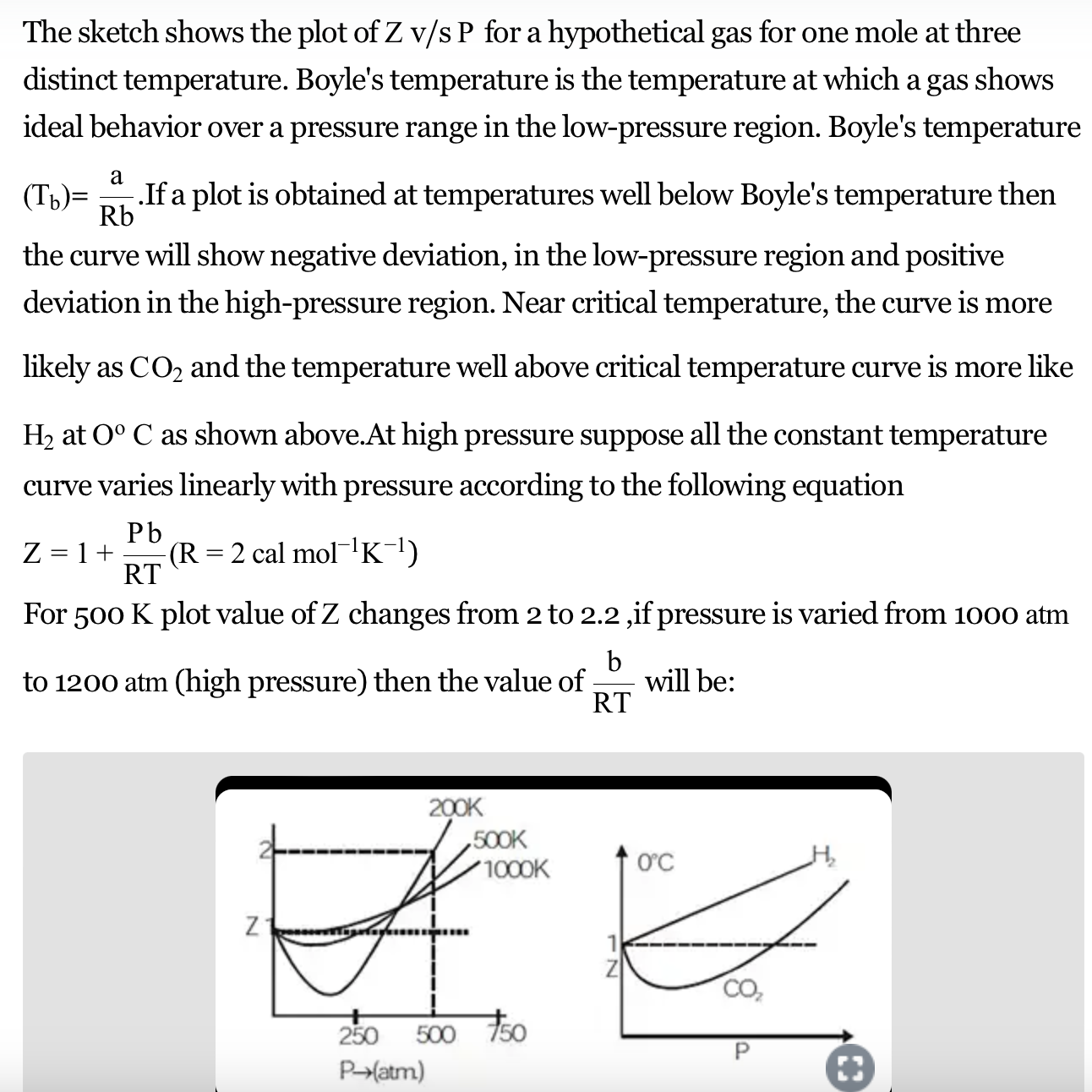
For a given gas, a graph is shown between compressibility factor
$ 20.00 · 4.9 (354) · In stock

By the graph, at T2, the gas does not deviate from ideal gas behaviour at low pressure (Z = 1).Hence, T2 = TBT2 = TB (Boyle's temperature)T1 > TB (Here, Z > 1)This implies that T3 B and TB > TC(Here, Z So, T1 and T2 must be above TC.
Solved The graph of compressibility factor (Z)v/sP for 1 mol

physical chemistry - Is the compressibility factor smaller or greater than 1 at low temperature and high pressure? - Chemistry Stack Exchange
The effect of Pressure on Temperature-Compressibility Factor diagram
JEE Advanced Practice Test- 2 Free MCQ Practice Test with Solutions - JEE

Deviation Of Real Gas From Ideal Gas Behavior

Real-gas z-factor, as attributed to Standing and Katz, 9 plotted

Compressibility Factor Z Important Concepts and Tips for JEE Main

The graph of compressibility factor (Z) :vs: P one mole of a real gas is shown in following diagram. The graph is plotted constant temperature 273 K. If the slope of graph

Plot of experimental measurements of the z-factor

1.3 Deviation from ideal gas behaviour

Non-Ideal Gas Behavior Chemistry: Atoms First
The graph of compressibility factor (Z) vs. P for one mole of a real gas ..

The following graph is plotted between compressibility factor Z versus pressure of a gas at different temperatures.Which of the following statements is /are correct?