4. A container contains 32 g of O2 at a temperature TThe pressure
$ 17.99 · 4.7 (529) · In stock
4. A container contains 32 g of O2 at a temperature TThe pressure of the gas is P. An identical containercontaining 4 g of H2 at a temperature 2T has apressure of(1) 8P(3) P(2) 4P(4) P18r cnstant
4- A container contains 32 g of O2 at a temperature TThe pressure of the gas is P- An identical containercontaining 4 g of H2 at a temperature 2T has apressure of-1- 8P-3- P-2- 4P-4- P18r-cnstant

A container contains 32 g of O2 at a temperature T. The pressure of the ..
68.A closed container has 2 non reactive gases neon and oxygen with tgeir partial pressure ratio 3:2 . If the atomic mass of Ne = 20.2 u and molecular mass of O2
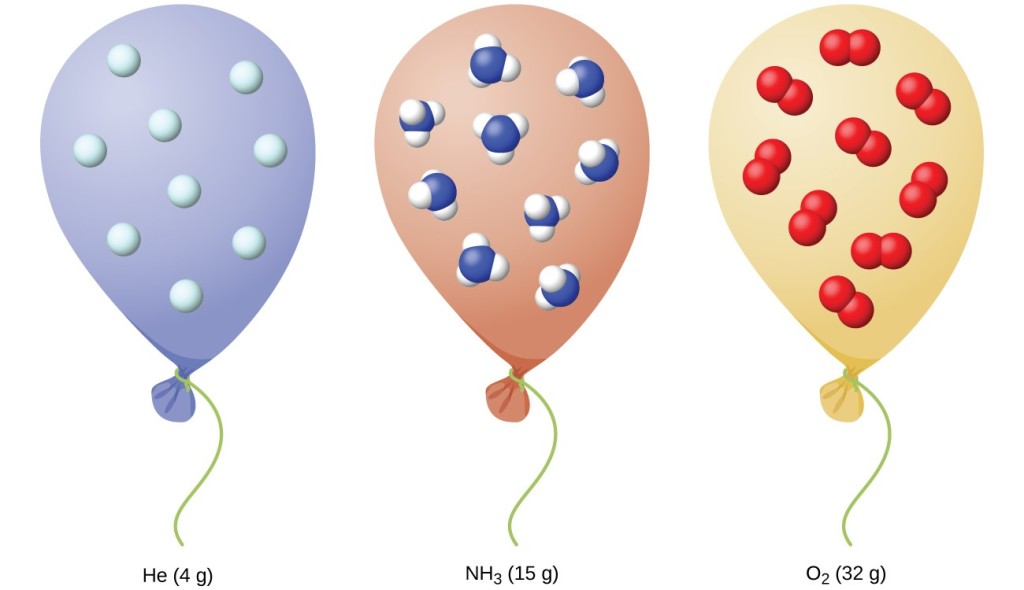
8.2: Relating Pressure, Volume, Amount, and Temperature: The Ideal Gas Law
What pressure is exerted by the mixture of 2.0g of hydrogen and 8.0 g of nitrogen at 273k in a 10l vessel? - Quora

8.2: Relating Pressure, Volume, Amount, and Temperature: The Ideal Gas Law
What is the amount of oxygen gas (O2) in a cylinder of 30l, if the pressure is 20atm at 30°C? - Quora

Maximum density of H,O is the temperature : (2) 39.2°F (1) 32°F
The Ideal Gas Law - Video Tutorials & Practice Problems

A sealed container contains a mixture of oxygen and nitrogen gas.The ratio is .The ratio is
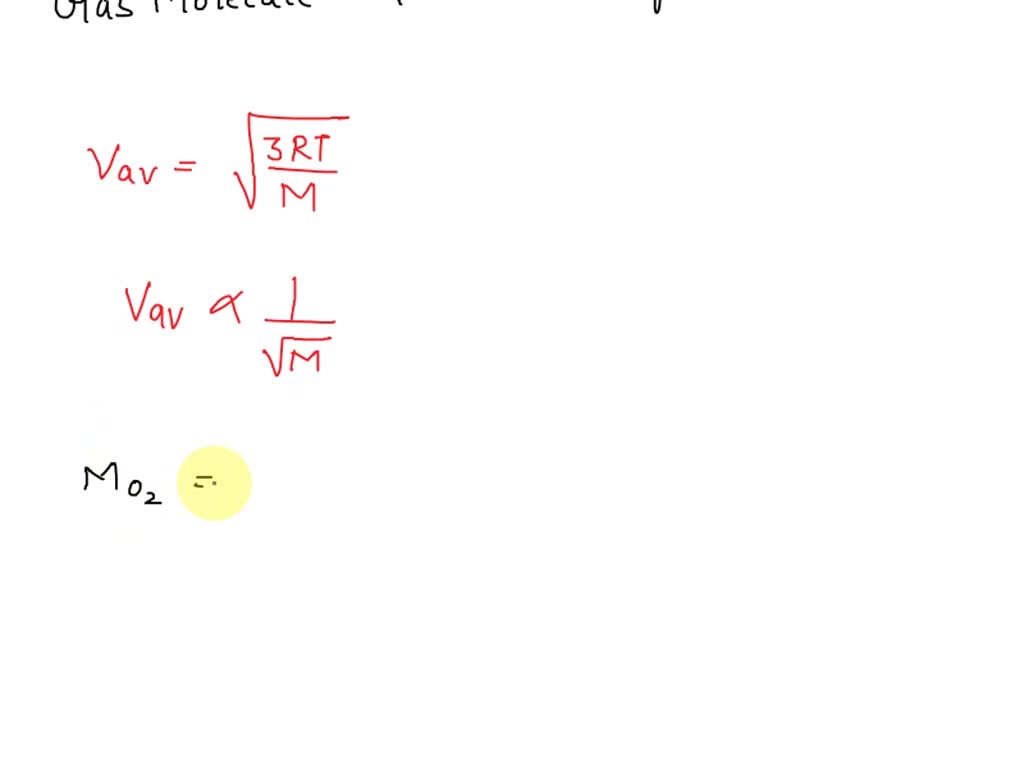
SOLVED: A container is filled with a mixture of helium and oxygen gases. A thermometer in the container indicates that the temperature is 22°C. Which gas molecules have the greater average speed?
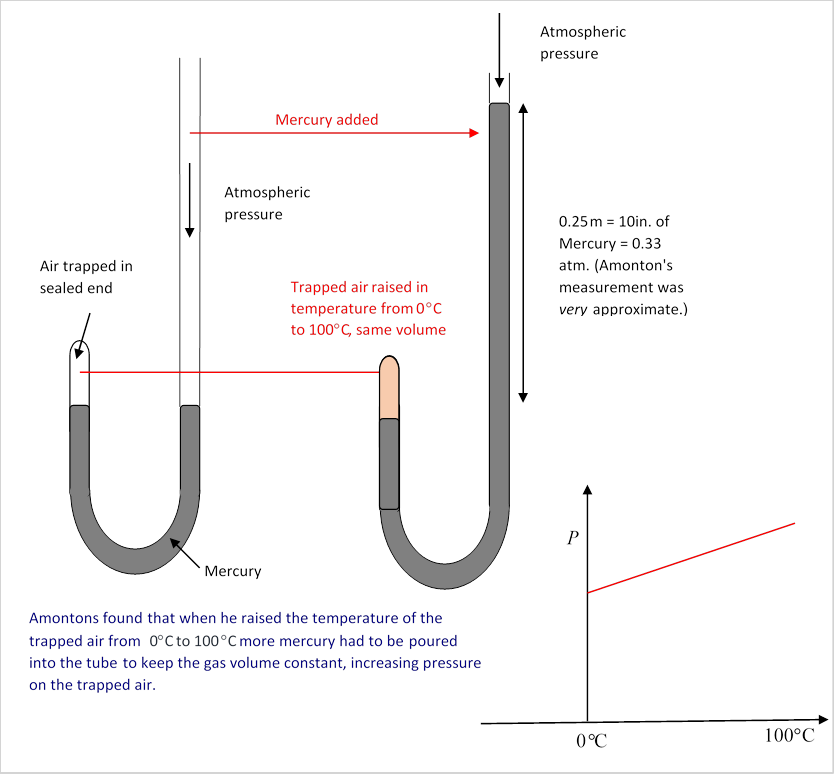
Gas Law and Avogadro
What pressure is exerted by the mixture of 2.0g of hydrogen and 8.0 g of nitrogen at 273k in a 10l vessel? - Quora
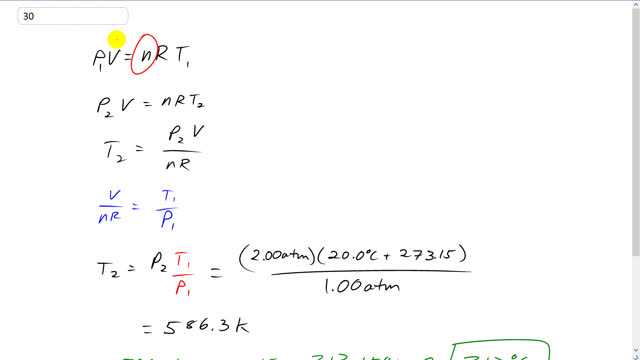
Giancoli 7th Edition, Chapter 13, Problem 30

A vessel X contains 1 mole of O2 gas (molar mass 32) a temperature and pressure P. Another identical vessel Y contains one mole of He gas (molar mass 4) temperature 27