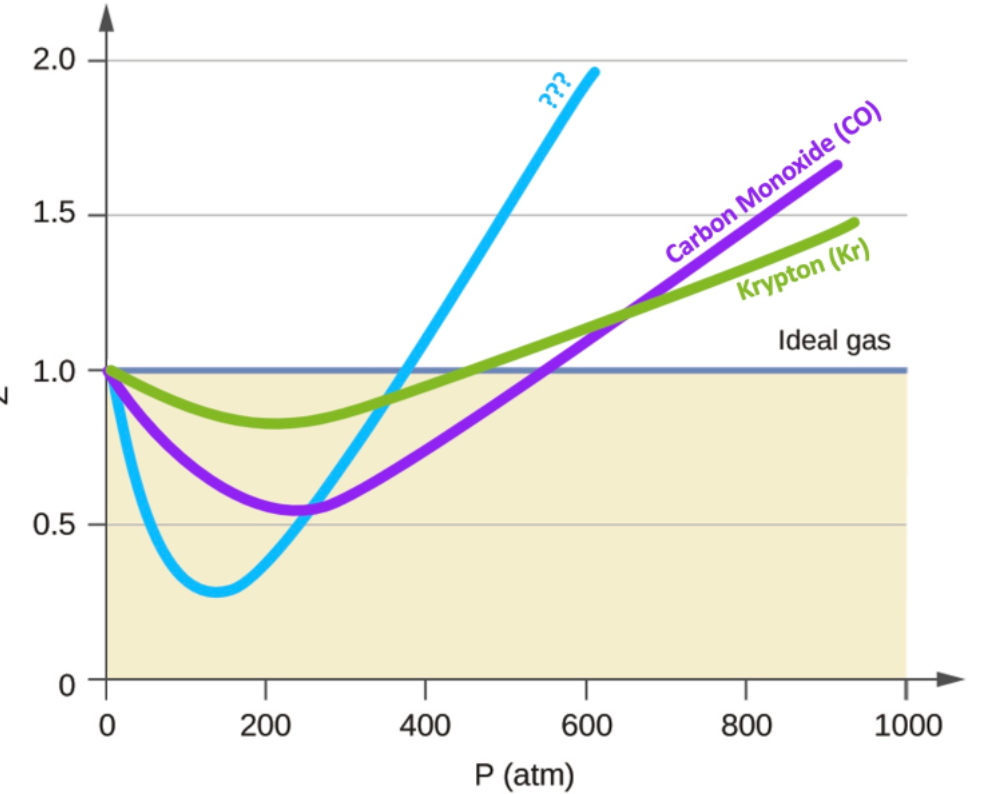
Compressibility factor Z = PV / nRT is plotted against pressure as
$ 17.99 · 4.6 (657) · In stock

Compressibility factor Z = PV / nRT is plotted against pressure as shown below:What is the correct order for the liquefiability of the gases shown in the above graph? A. CO 2
Compressibility factor Z - PV - nRT is plotted against pressure as shown below-What is the correct order for the liquefiability of the gases shown in the above graph- A- CO 2- CH 4- N 2- H 2B- H 2- CH 4- N 2- CO 2C- CH 4- H 2- N 2- CO 2D- H 2- N 2- CH 4- CO 2

physical chemistry - Why do some gases have lower value of Z for a particular pressure? - Chemistry Stack Exchange

Compressibility Factor Calculator - File Exchange - MATLAB Central
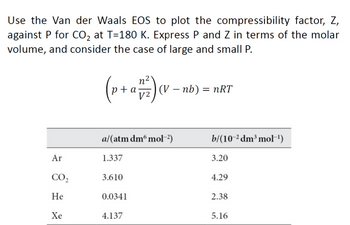
Answered: Use the Van der Waals EOS to plot the…

y factor Compressibility factor 2 V is plotted agalnst pressure RT What is the correct order of correct order of liquet ability of the gases shown in the above graphi (5) Hz

Introduction Ideal Gas Equation - ppt download
Essential Pharma Documents: 1205: Properties of Gases
Solved Below is a plot of the compressibility factor (Z) as

Compressibility factorZPVnRTis plotted againstpressure What is the correct order of liquefiability of the gases shown in the above graph

Gas Compressibility - an overview

PVCompressibility factor Z=-isnRTplotted against pressure: What isthe correct order of liquefiabilityof the

The given graph represents the variation of Z (compressibility factor = \[\dfrac{{PV}}{{nRT}}\] ) versus P, for three real gases A, B and C. Identify the only incorrect statement.

The given graph represents the variation of Z (compressibility factor) vs. P three real gases A, B and C. Identify the correct statementFor the gas A, a=0 and its dependence on P

The graph of compressibility factor (Z) vs. P for one mole of a real gas is shown in following
1.5 Real Gases and the Virial Equation - Mail
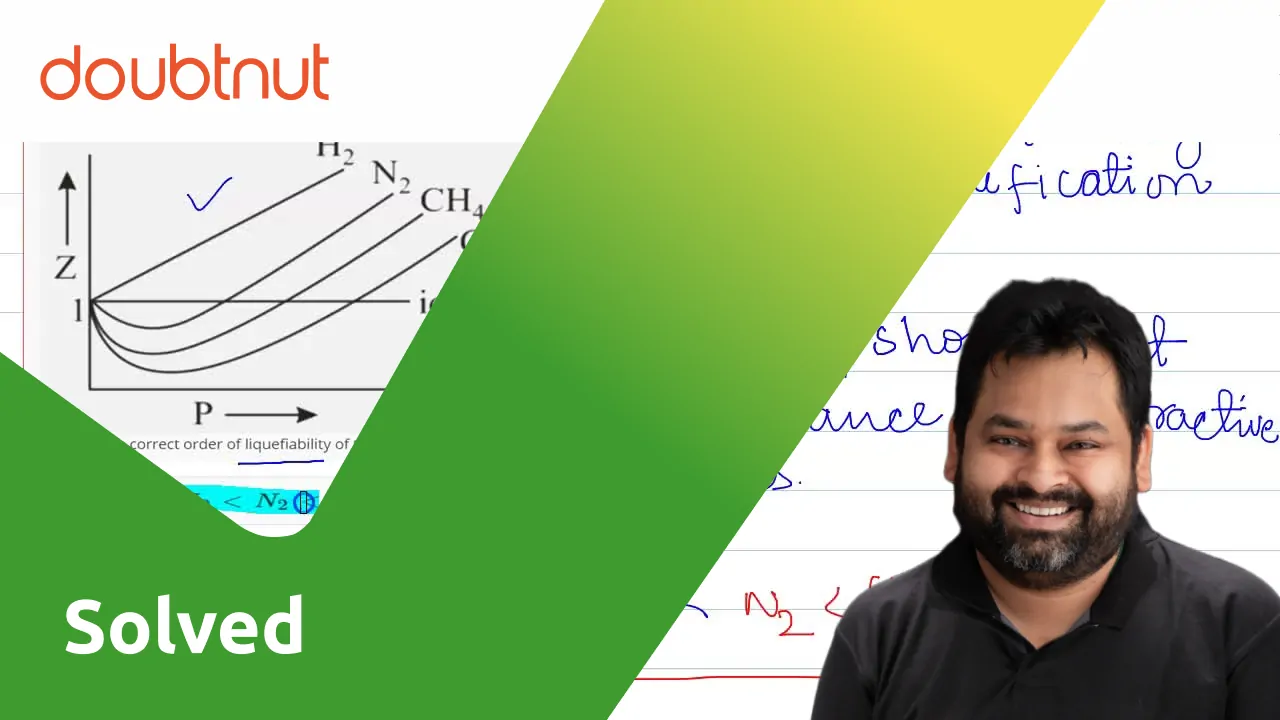
Compressibility factor (Z=(PV)/(nRT)) is plotted against pressure